
How water filters work
Thanks largely to an unusual molecular structure, water is amazingly good at dissolving things. (We look at this in more detailed in our main article on water.) Sometimes that's helpful: if you want to bust the dust from your jeans, simply throw them in your washing machine with some detergent and the water and soap will pull the muck away like a magnet. But there's clearly a downside to this too. All of our water constantly circulates through the environment in what's known as the water cycle. One minute it's rushing through a river or drifting high in a cloud, the next it's streaming from your faucet (tap), sitting in a glass on your table, or flushing down your toilet. How do you know the water you're about to drink—with its brilliant ability to attract and dissolve dirt—hasn't picked up all kinds of nasties on its journey through Earth and atmosphere? If you want to be sure, you can run it through a water filter.
Water filters use two different techniques to remove dirt. Physical filtration means straining water to remove larger impurities. In other words, a physical filter is a glorified sieve—maybe a piece of thin gauze or a very fine textile membrane. (If you have an electric kettle, you probably have a filter like this built into the spout to remove particles of limescale.) Another method of filtering, chemical filtration, involves passing water through an active material that removes impurities chemically as they pass through.

Four types of water filters
There are four main types of filtration and they employ a mixture of physical and chemical techniques.
Activated carbon

The most common household water filters use what are known as activated carbon granules (sometimes called active carbon or AC) based on charcoal (a very porous form of carbon, made by burning something like wood in a reduced supply of oxygen). Charcoal is like a cross between the graphite "lead" in a pencil and a sponge. It has a huge internal surface area, packed with nooks and crannies, that attract and trap chemical impurities through a process called adsorption (where liquids or gases become trapped by solids or liquids). But while charcoal is great for removing many common impurities (including chlorine-based chemicals introduced during waste-water purification, some pesticides, and industrial solvents), it can't cope with "hardness" (limescale), heavy metals(unless a special type of activated carbon filter is used), sodium, nitrates, fluorine, or microbes. The main disadvantage of activated carbon is that the filters eventually clog up with impurities and have to be replaced. That means there's an ongoing (and sometimes considerable) cost.
Reverse osmosis
Reverse osmosis means forcing contaminated water through a membrane (effectively, a very fine filter) at pressure, so the water passes through but the contaminants remain behind.
A closer look at reverse osmosis
If you've studied biology, you've probably heard of osmosis. When you have a concentrated solution separated from a less concentrated solution by a semi-permeable membrane (a kind of filter through which some things can pass, but others can't), the solutions try to rearrange themselves so they're both at the same concentration.
Wait, it's simpler than it sounds!
Suppose you have a sealed glass bottle full of very sugary water and you stand it inside a big glass jug full of less sugary water. Nothing will happen. But what if the bottle is actually a special kind of porous plastic through which water (but not sugar) can travel? What happens is that water moves from the outer jug through the plastic (effectively, a semi-permeable membrane) into the bottle until the sugar concentrations are equal. The water moves all by itself under what's called osmotic pressure.
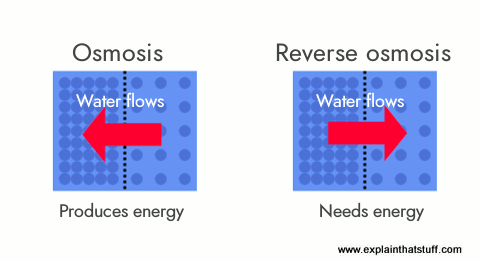
That's osmosis, so what about reverse osmosis? Suppose you take some contaminated water and force it through a membrane to make pure water. Effectively, you're making water go in the opposite direction to which osmosis would normally make it travel (not from a less-concentrated solution to a more-concentrated solution, as in osmosis, but from a more-concentrated solution to a less-concentrated solution).
Since you're making the water move against its natural inclination, reverse osmosis involves forcing contaminated water through a membrane under pressure—and that means you need to use energy. In other words, reverse-osmosis filters have to use electrically powered pumps that cost money to run. Like activated charcoal, reverse osmosis is good at removing some pollutants (salt, nitrates, or limescale), but less effective at removing others (bacteria, for example). Another drawback is that reverse osmosis systems produce quite a lot of waste-water—some waste four or five liters of water for every liter of clean water they produce.
Here's what a reverse osmosis filter unit looks like in practice, shown in cutaway. Unfiltered water (blue pipe) is pumped into a purification unit (gray) and passes through a plastic, semi-permeable membrane (yellow) made (in this case) of cellulose acetate. Clean water flows out through the red pipe; impurities flush away through the green pipe:

Ion exchange
Ion-exchange filters are particularly good at "softening" water (removing limescale). They're designed to split apart atoms of a contaminating substance to make ions (electrically charged atoms with too many or too few electrons). Then they trap those ions and release, instead, some different, less troublesome ions of their own—in other words, they exchange "bad" ions for "good" ones.

How do they work? Ion exchange filters are made from lots of zeolite beads containing sodium ions. Hard water contains magnesium and calcium compounds and, when you pour it into an ion-exchange filter, these compounds split apart to form magnesium and calcium ions. The filter beads find magnesium and calcium ions more attractive than sodium, so they trap the incoming magnesium and calcium ions and release their own sodium ions to replace them. Without the magnesium and calcium ions, the water tastes softer and (to many people) more pleasant. However, the sodium is simply a different form of contaminant, so you can't describe the end product of ion-exchange filtration as "pure water" (the added sodium can even be problematic for people on low-sodium diets). Another disadvantage of ion-exchange filtration is that you need to recharge the filters periodically with more sodium ions, typically by adding a special kind of salt. (This is why you have to add "salt" to dishwashers, from time to time: the salt recharges the dishwasher's water softener and helps to prevent a gradual build-up of limescale that can damage the machine.)
Distillation
One of the simplest ways to purify water is to boil it, but although the heat kills off many different bacteria, it doesn't remove chemicals, limescale, and other contaminants. Distillation goes a step further than ordinary boiling: you boil water to make steam, then capture the steam and condense (cool) it back into water in a separate container. Since water boils at a lower temperature than some of the contaminants it contains (such as toxic heavy metals), these remain behind as the steam separates away and boils off. Unfortunately, though, some contaminants (including volatile organic compounds or VOCs) boil at a lower temperature than water and that means they evaporate with the steam and aren't removed by the distillation process.
On balance
You can see that different types of filtration remove different pollutants—but there's no single technique that removes all the contaminants from water. That's why many home water-filter systems use two or more of these processes together. If you're looking for a home water filter, tread carefully. Bear in mind that you won't necessarily remove all the nasties. Remember, too, that most water filters require some kind of ongoing cost and, without regular maintenance to keep them working properly, can leave your water in worse shape than it was to begin with!







No comments:
Post a Comment